Friedel–Crafts functionalization of the cyclopentadienyl ligand in buckymetallocenes†
Abstract
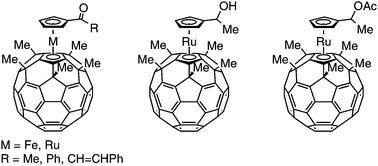
Acylated buckyferrocene and ruthenocene, Fe(η5-C60Me5)(η5-C5H4COR) (R = Me, Ph, and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh) and Ru(η5-C60Me5)(η5-C5H4COR) (R = Me and Ph), were obtained by Friedel–Crafts acylation of the parent buckymetallocenes with the corresponding acid chlorides and aluminum chloride in carbon disulfide at ambient temperature. The electron withdrawing and sterically hindered nature of the acyl groups were revealed by X-ray crystallography, infrared spectroscopy and electrochemical measurements. The possibility of further derivatizing the acylated products was illustrated by the conversion of the acetyl buckyruthenocene into the corresponding hydroxy and acetoxy compounds Ru(η5-C60Me5)(η5-C5H4CH(OH)Me) and Ru(η5-C60Me5)(η5-C5H4CH(OAc)Me).
CHPh) and Ru(η5-C60Me5)(η5-C5H4COR) (R = Me and Ph), were obtained by Friedel–Crafts acylation of the parent buckymetallocenes with the corresponding acid chlorides and aluminum chloride in carbon disulfide at ambient temperature. The electron withdrawing and sterically hindered nature of the acyl groups were revealed by X-ray crystallography, infrared spectroscopy and electrochemical measurements. The possibility of further derivatizing the acylated products was illustrated by the conversion of the acetyl buckyruthenocene into the corresponding hydroxy and acetoxy compounds Ru(η5-C60Me5)(η5-C5H4CH(OH)Me) and Ru(η5-C60Me5)(η5-C5H4CH(OAc)Me).
- This article is part of the themed collection: Organometallic and coordination chemistry of carbon nanomaterials

 Please wait while we load your content...
Please wait while we load your content...