Interactions of carbon nanotubes and gold nanoparticles: the effects of solvent dielectric constant and temperature on controlled assembly of superstructures†
Abstract
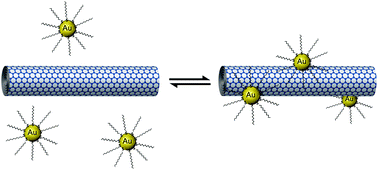
The effects of solvent dielectric constant and temperature on the non-covalent interactions between gold nanoparticles and carbon nanotubes have been explored. Our experiments have shown that fewer nanoparticles are adsorbed onto nanotubes in high dielectric assembly environments. This has been correlated with an increase in the differential capacitance of nanoparticles relative to the bulk solvent resulting in more local charge on nanoparticles and thus heightened repulsive electrostatic interactions in higher polarity organic solvents. Furthermore, our temperature-dependent measurements have demonstrated for the first time that (i) the apparent activation barrier to adsorption of nanoparticles on nanotubes of Ea = 9.6 kJ mol−1 lies clearly within the range expected for non-covalent interactions and (ii) the adsorption of nanoparticles onto nanotubes is reversible and may represent an equilibrium process sensitive to temperature according to Le Chatelier's principle. Thus, we further demonstrate that modulation of non-covalent interactions can be harnessed for the precision derivatisation of nanocarbons with noble metals.
- This article is part of the themed collection: Organometallic and coordination chemistry of carbon nanomaterials

 Please wait while we load your content...
Please wait while we load your content...