Speciation and the structure of lead(ii) in hyper-alkaline aqueous solution†
Abstract
The identity of the predominating lead(II) species in hyper-alkaline aqueous solution has been determined by Raman spectroscopy, and ab initio quantum chemical calculations and its structure has been determined by EXAFS. The observed and calculated Raman spectra for the [Pb(OH)3]− complex are in agreement while they are different for two-coordinated complexes and complexes containing Pb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds. Predicted bond lengths are also consistent with the presence of [Pb(OH)3]– and exclude the formation of Pb
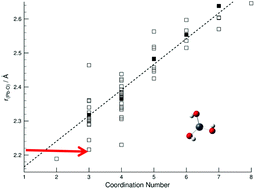
O double bonds. Predicted bond lengths are also consistent with the presence of [Pb(OH)3]– and exclude the formation of Pb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond(s). These observations together with experimentally established analogies between lead(II) and tin(II) in hyper-alkaline aqueous solutions suggest that the last stepwise hydroxido complex of lead(II) is [Pb(OH)3]−. The Pb–O bond distance in the [Pb(OH)3]− complex as determined is remarkably short, 2.216 Å, and has low symmetry as no multiple back-scattering is observed. The [Pb(OH)3]− complex has most likely trigonal pyramidal geometry as all reported three-coordinated lead(II) complexes in the solid state. From single crystal X-ray data, the bond lengths for O-coordinated lead(II) complexes with low coordination numbers are spread over an unusually wide interval, 2.216–2.464 Å for N = 3. The Pb–O bond distance is at the short side and within the range of three coordinated complexes, as also observed for the trihydroxidostannate(II) complex indicating that the hydroxide ion forms short bonds with d10s2 metal ions with occupied anti-bonding orbitals.
O double bond(s). These observations together with experimentally established analogies between lead(II) and tin(II) in hyper-alkaline aqueous solutions suggest that the last stepwise hydroxido complex of lead(II) is [Pb(OH)3]−. The Pb–O bond distance in the [Pb(OH)3]− complex as determined is remarkably short, 2.216 Å, and has low symmetry as no multiple back-scattering is observed. The [Pb(OH)3]− complex has most likely trigonal pyramidal geometry as all reported three-coordinated lead(II) complexes in the solid state. From single crystal X-ray data, the bond lengths for O-coordinated lead(II) complexes with low coordination numbers are spread over an unusually wide interval, 2.216–2.464 Å for N = 3. The Pb–O bond distance is at the short side and within the range of three coordinated complexes, as also observed for the trihydroxidostannate(II) complex indicating that the hydroxide ion forms short bonds with d10s2 metal ions with occupied anti-bonding orbitals.

 Please wait while we load your content...
Please wait while we load your content...