The KCaSrTa5O15 photocatalyst with tungsten bronze structure for water splitting and CO2 reduction†
Abstract
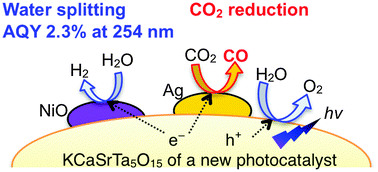
KCaSrTa5O15 with tungsten bronze structure and a band gap of 4.1 eV showed activity for water splitting without cocatalysts. The activity was improved by loading the NiO cocatalyst. The apparent quantum yield of optimized NiO-loaded KCaSrTa5O15 was 2.3% at 254 nm for water splitting. When CO2 gas was bubbled into the reactant aqueous solution, Ag cocatalyst-loaded KCaSrTa5O15 produced CO and H2 as reduction products of CO2 and H2O, respectively, and O2 as an oxidation product of H2O. The carbon source of CO was confirmed to be CO2 molecules by using 13CO2. The ratio of the number of electrons to that of holes calculated from the amounts of products (CO, H2 and O2) was almost unity. Additionally, the ratio of the turnover number of electrons consumed for CO production to the total number of an Ag atom of the cocatalyst that was the active site for CO2 reduction was 8.6 at 20 h. These results indicate that water was consumed as an electron donor for this photocatalytic CO2 reduction in an aqueous medium. Thus, KCaSrTa5O15 with tungsten bronze structure has arisen as a new photocatalyst that is active for water splitting and CO2 reduction utilizing water as an electron donor.

 Please wait while we load your content...
Please wait while we load your content...