On the self-assembly of a tryptophan labeled deoxycholic acid†
Abstract
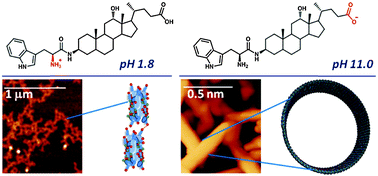
Self-assembly of peptides and bile acids has been widely investigated because of their biological role and their potential as a tool for the preparation of nanostructured biomaterials. We herein report both the synthesis and the self-association behavior of a compound that combines the aggregation properties of bile acid- and amino acid-based molecules. The derivative has been prepared by introducing a L-tryptophan residue into the C-3 position of the deoxycholic acid skeleton and resulted in an amphoteric fluorescent labeled bile acid that shows a pH-dependent self-assembly. Under alkaline conditions it assembles into 28 nm diameter tubules, thus showing a completely different behavior compared to the precursor bile acid, which forms micelles under similar conditions. Upon heating the tubules break and turn into micelles, leading to an increase in the exposure to water of the tryptophan residue. On the other hand, in acidic solutions it aggregates into elongated micelles that further self-assemble forming a gel network, when an electrolyte is added.

 Please wait while we load your content...
Please wait while we load your content...