Topological analyses and small-world patterns of hydrogen bond networks in water + t-butanol, water + n-butanol and water + ammonia mixtures†
Abstract
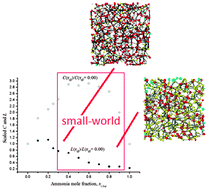
Cluster (or island) statistics and topological statistical mechanics based properties were employed in the analyses of hydrogen bond (H-bond) networks of t-butanol, n-butanol and ammonia aqueous solutions. These networks were generated from equilibrated Monte Carlo simulations at mixture compositions covering the entire range of miscibility and a fine grid of points around the topological transitions. We found that these H-bond networks changed from a percolation regime in water rich mixtures to a non-percolating behavior at non-aqueous component rich compositions. Topological analysis of local (clustering coefficients, average degrees) semi-global (path lengths) and global (spectral densities) properties indicated the presence of small-world patterns for the H-bond networks in mixtures at mole fraction compositions larger than ca. 0.6. These small-world patterns are characterized by highly clustered networks with small path lengths. Spectral densities show high order moment contributions that correlate with small-world patterns, thus corroborating the robustness of these statistical mechanics based topological analyses. The degree distributions of these networks were partially rationalized by the differences in the water–water and solute–solute H-bonds.

 Please wait while we load your content...
Please wait while we load your content...