Understanding the growth and photoelectrochemical properties of mesocrystals and single crystals: a case of anatase TiO2†
Abstract
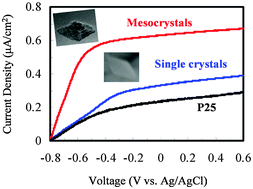
Anatase TiO2 mesocrystals and single crystals with dominant {101} facets were successfully synthesized without any additives using titanate nanowires as precursors under solvothermal and hydrothermal conditions, respectively. It is proposed that the oriented self-assembly process for the formation of TiO2 mesocrystals was controlled by the same thermodynamic principle as that of single crystals in this simple reaction system. Furthermore, the TiO2 mesocrystals were applied in photoelectrochemical (PEC) water splitting and demonstrated much enhanced photocurrent, almost 191% and 274% compared with that of TiO2 single crystals and commercial P25, respectively. Electrochemical impedance measurements under illumination revealed that the photocurrent increase was largely ascribed to the effective charge separation of electron–hole pairs and fast interfacial charge transfer. This could be attributed to the intrinsic characteristics of the mesostructured TiO2 composed of highly oriented nanocrystal subunits offering few grain boundaries, nanoporous nature and a short transport distance.

 Please wait while we load your content...
Please wait while we load your content...