Aggregation kinetics of single-walled carbon nanotubes investigated using mechanically wrapped multinuclear complexes: probing the tube–tube repulsive barrier†
Abstract
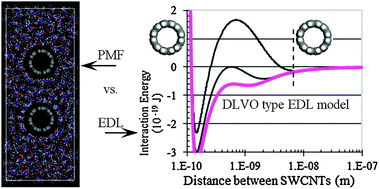
The rational design of supraparticle assemblies requires a detailed understanding of directed assembly processes. The stability of dispersions of nanoscale materials, like single-walled carbon nanotubes (SWCNTs), is still not fully understood, nor are the mechanisms of aggregation and assembly. A detailed balance of attractive van der Waals type interactions with various repulsive barrier mechanisms is needed to control the assembly of industrially viable and functional hybrid-nanoscale supraparticles. We report a detailed study of SWCNT dispersion stability and aggregation kinetics as a function of the nature of the coagulant used in various solvent systems. We explore three classes of coagulants that vary in charge, size, shape, solvation energy, and the ability to bind to the SWCNTs. We use these kinetic data to assess the tube–solvent–coagulant–tube interactions. We compare the relative contributions from two types of repulsive barriers. We find that tube-mediated structured solvent around the SWCNTs does not sufficiently describe our measured kinetic data. A DLVO type, electrical double layer repulsion is used to rationalize our observations. The data presented in this paper require a more detailed theoretical understanding of the physico-chemical environment near nanoparticle surfaces such as aggregating SWCNTs.

 Please wait while we load your content...
Please wait while we load your content...