Large-scale synthesis and formation mechanism study of basic aluminium sulfate microcubic crystals†
Abstract
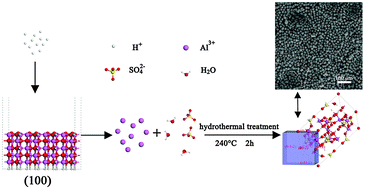
Cube-like basic aluminium sulfate crystals were prepared by a facile template-free hydrothermal strategy. The microstructures, morphologies and textural properties of as-synthesized material were characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy (FE-SEM) and transmission electron microscopy. X-ray crystallography reveals that cubic basic aluminium sulfate possesses a single crystal nature. Chemical formation mechanism studies of sulfuric acid with γ-AlOOH were performed using a combined experimental and computational approach. Time dependent experiments reveal that formation of basic aluminium sulfate is based on the dissolution–recrystallization process, and the source of Al3+ is from the dissolution of γ-AlOOH at high H+ concentration. Moreover, the quantum mechanical calculations reveal that dramatic structural changes occurred in the (100) plane at high H+ concentration, which is inferred to be the initiation of the source of Al3+. Meanwhile, surface energy calculations can well explain the exposed plane of basic aluminium sulfate microcubes, which are consistent with the XRD results. Besides, equations to quantitatively describe the relationship between the molar amount of H+ and the final phase are proposed, which has been confirmed by experimental results.

 Please wait while we load your content...
Please wait while we load your content...