The strong catalytic effect of Pb(ii) on the oxygen reduction reaction on 5 nm gold nanoparticles
Abstract
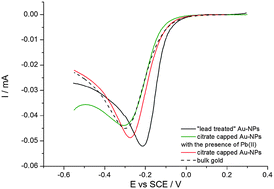
Citrate-capped gold nanoparticles (AuNPs) of 5 nm in diameter are synthesized via wet chemistry and deposited on a glassy carbon electrode through electrophoresis. The kinetics of the oxygen reduction reaction (ORR) on the modified electrode is determined quantitatively in oxygen-saturated 0.5 M sulphuric acid solution by modelling the cathode as an array of interactive nanoelectrodes. Quantitative analysis of the cyclic voltammetry shows that no apparent ORR electrocatalysis takes place, the kinetics on AuNPs being effectively the same as on bulk gold. Contrasting with the above, a strong ORR catalysis is found when Pb2+ is added to the oxygen saturated solution or when the modified electrode is cycled in lead alkaline solution such that lead dioxide is repeatedly electrodeposited and stripped off on the nanoparticles. In both cases, the underpotential deposition of lead on the gold nanoparticles is found to be related to the catalysis.

 Please wait while we load your content...
Please wait while we load your content...