Conformational changes in Cmethyl-resorcinarene pyridine N-oxide inclusion complexes in the solid state†
Abstract
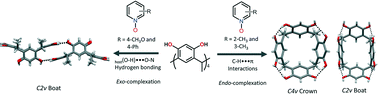
Aromatic N-oxides interact with Cmethyl-resorcinarene resulting in marked changes in the conformation of the host resorcinarene. In the solid state, 2- and 3-methylpyridine N-oxides form pseudo-capsular 2 : 2 endo host–guest complexes with Cmethyl-resorcinarene stabilized by C–H⋯π interactions. The Cmethyl-resorcinarene·2-methylpyridine N-oxide complex has a C4v crown conformation, while the Cmethyl-resorcinarene·3-methylpyridine N-oxide complex has a slightly open C2v boat conformation. On the contrary, other para-substituted and benzo-fused pyridine N-oxides form only exo complexes with Cmethyl-resorcinarene. In the exo complexes, the asymmetry of the guest, conformational flexibility and preferred inter-host hydrogen bonding of Cmethyl-resorcinarenes exclude endo complexation. All the exo complexes form robust 1-D hydrogen bonded chains between the host hydroxyl groups assisted by the guest N–O groups, resulting in a C2v boat conformation.
- This article is part of the themed collection: Form and Function of Molecular Cups and Capsules

 Please wait while we load your content...
Please wait while we load your content...