Insight into the mechanism of modulated syntheses: in situ synchrotron diffraction studies on the formation of Zr-fumarate MOF†
Abstract
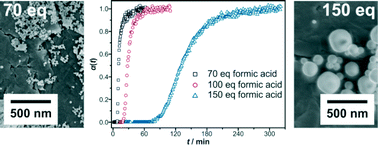
In this work, the formation of a Zr-based metal–organic framework (MOF), Zr-fumarate MOF (Zr-fum MOF), is studied in situ by energy-dispersive diffraction. The Zr-fum MOF can be synthesised in DMF as well as in water-based synthesis systems. In both cases, its formation requires modulation, i.e. a monocarboxylic acid which is used as the modulator has to be added to the synthesis mixture. In general, different mechanisms of modulation are possible, for example, deprotonation of the linker molecule (deprotonation modulation) or coordination modulation (wherein the molecules of the modulator compete with the linker molecules for the coordination sites at the inorganic building units). Independently of the specific mechanism, modulation often improves the reproducibility of the MOF synthesis and the crystallinity of the product and may be used to control crystal size and morphology. This study is the first to investigate the kinetics of modulated MOF syntheses with regard to coordination modulation. According to this concept, the addition of a modulator usually decelerates the reaction. Our kinetic investigations show that this is the case for the formation of Zr-fum MOF in the water-based synthesis with formic acid used as a modulator. On the contrary, the addition of formic acid to the DMF-based synthesis results in an accelerating effect. This unexpected effect can be attributed to a small amount of water present in formic acid. Correspondingly, the addition of water to the synthesis mixture also showed an accelerating effect. These investigations emphasise the subtle interplay of the different ingredients in a MOF synthesis. In the case of the Zr-fum MOF, both the modulator formic acid and the water content strongly affect the kinetics of crystallisation. Quantitative evaluation of the kinetic data using the Gualtieri equation provides additional insight into the mechanisms of coordination-modulated MOF formation reactions and excludes the idea of deprotonation modulation.

 Please wait while we load your content...
Please wait while we load your content...