Direct core functionalisation of naphthalenediimides by iridium catalysed C–H borylation†
Abstract
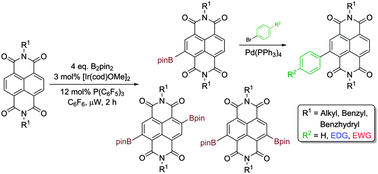
We report the first boron-substituted naphthalenediimides (NDIs), prepared by iridium catalysed C–H activation. When the NDI substrates bear N-benzyl substituents, the naphthyl NDI core is borylated in preference, suggestive of a directed borylation mechanism. Borylated NDIs are substrates for Suzuki–Miyaura couplings and borylation of an NDI bearing two inequivalent N-substituents has also been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...