Mechanochemical C–H bond activation: rapid and regioselective double cyclopalladation monitored by in situ Raman spectroscopy†
Abstract
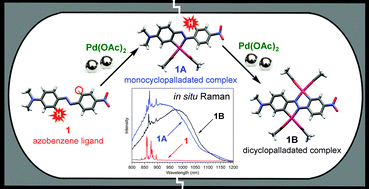
The first direct mechanochemical transition-metal-mediated activation of strong phenyl C–H bonds is reported. The mechanochemical procedure, resulting in cyclopalladated complexes, is quantitative and significantly faster than solution synthesis and allows highly regioselective activation of two C–H bonds by palladium(II) acetate in asymmetrically substituted azobenzene. Milling is monitored by in situ solid-state Raman spectroscopy which in combination with quantum-chemical calculations enabled characterization of involved reaction species, direct insight into the dynamics and reaction pathways, as well as the optimization of a milling process.

 Please wait while we load your content...
Please wait while we load your content...