Oxidative skeletal rearrangement of 1,1′-binaphthalene-2,2′-diamines (BINAMs) via C–C bond cleavage and nitrogen migration: a versatile synthesis of U-shaped azaacenes†
Abstract
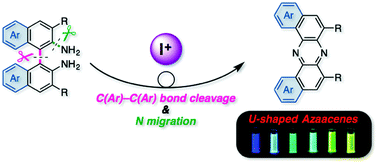
An oxidative skeletal rearrangement of 1,1′-binaphthalene-2,2′-diamines (BINAMs) that involves the cleavage of a strong C–C single bond of the binaphthalene unit and the nitrogen migration has been discovered. The unprecedented rearrangement enables access to a series of U-shaped azaacenes otherwise difficult to prepare in a selective manner by classical methods. Moreover, physicochemical properties of the unique azaacenes have been comprehensively investigated.

 Please wait while we load your content...
Please wait while we load your content...