Boron–nitrogen substituted perylene obtained through photocyclisation†
Abstract
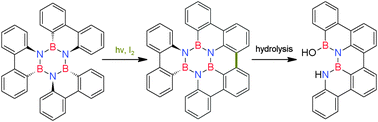
A BN substituted hexabenzotriphenylene (B3N3) closes one C–C-bond upon irradiation with light of 280–400 nm in the presence of iodine to yield a phenanthrene annelated B3N3 tribenzoperylene. Upon hydrolysis a B2N2 dibenzoperylene is obtained.

 Please wait while we load your content...
Please wait while we load your content...