Direct N9-arylation of purines with aryl halides†
Abstract
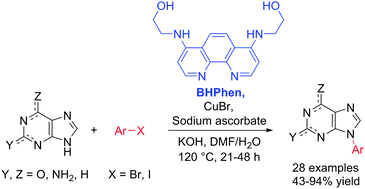
An efficient method for N-arylation of purines is reported. The N-arylation is catalysed by Cu(I) and 4,7-bis(2-hydroxyethylamino)-1,10-phenanthroline (BHPhen) in aqueous DMF or ethanol. The reaction generally proceeds with high selectivity for the N9-position.

 Please wait while we load your content...
Please wait while we load your content...