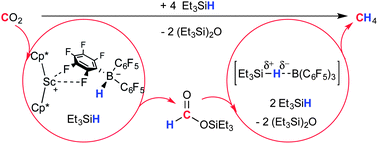
This work was directed at studying the capability of structurally defined, strongly Lewis-acidic metal centers to effect catalytic reductive fixation of the small molecule substrate CO2. Exposing solutions or solid samples of the ion pair [Cp*2Sc][HB(C6F5)3] 1CIP, in which the highly electrophilic decamethyl-scandocene cation and [HB(C6F5)]− as a potentially reactive source of hydride equivalents are associated, to CO2 selectively produces ion pair [Cp*2Sc][HCO2B(C6F5)3] 2CIP. The results of solution and solid state structural analysis of 2CIP imply ionic association of [Cp*2Sc]+ and [HCO2B(C6F5)3]− rather than B(C6F5)3-adduct formation to neutral Cp*2Sc-formate. In the presence of B(C6F5)3 co-catalyst and excess triethylsilane, the formation of 2CIP from 1CIP initiates the catalytic deoxygenative hydrosilation of CO2 to CH4. The roles of ion pairs 1 and 2, borane co-catalyst, and silane in the catalytic reaction were studied mechanistically by NMR spectroscopy. Intermediately formed 3,3,7,7-tetraethyl-3,7-disila-4,6-dioxanonane product was found to exert an accelerating effect on the overall reaction rate by promoting [HCO2B(C6F5)3]− dissociation to give 2SIP through formation of separated ion pairs [Cp*2Sc(κ2-(Et3SiO)2CH2)][HCO2{B(C6F5)3}n], n = 1, 2. DFT calculations show that the formation 2CIP from the reaction of 1CIP with CO2 is exoergic and without significant energy barriers. This work lays the basis for future studies of reactive ion pairs of this kind in the context of small molecule chemistry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...