Electrochemical insertion of Li into nanocrystalline MnFe2O4: a study of the reaction mechanism†
Abstract
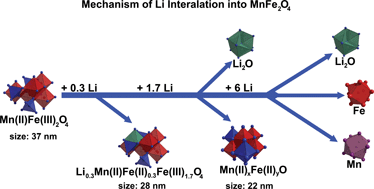
The study of the mechanism of Li insertion into nanosized partially inverse spinel MnFe2O4 applying X-ray diffraction, in situ quick X-ray absorption spectroscopy, Mössbauer spectroscopy, high resolution transmission electron microscopy, 7Li MAS NMR, and electrochemical measurements yields a comprehensive picture of the individual steps occurring during Li uptake. At the very early beginning of the reaction Fe3+ on the tetrahedral site is reduced and moves to empty octahedral sites. Increasing the amount of Li to 0.7 per MnFe2O4, further Fe3+ is reduced and Mn2+ residing on the tetrahedral site moves to empty octahedral sites thus forming a defect NaCl-type structure. At least for 2 Li per MnFe2O4 reflections of the spinel disappeared in the X-ray powder pattern and only those of a monoxide are observed. No indications were found for a phase separation and Fe and Mn are homogeneously distributed over the sample. Further Li uptake leads to a stepwise conversion of the material and after insertion of 8 Li/MnFe2O4 only nanosized Mn, Fe, and Li2O are detected. After a capacity loss at the beginning of Li insertion, a constant capacity of about 266 mA h g−1 is reached after 100 cycles discharging–charging the material.

 Please wait while we load your content...
Please wait while we load your content...