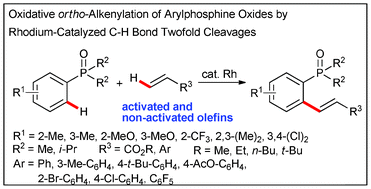
Oxidative ortho-alkenylation of arylphosphine oxides by rhodium-catalyzed C–H bond twofold cleavage†
Abstract
An efficient Rh-catalyed oxidative ortho-alkenylation is reported from the reaction of arylphosphine oxides with activated and non-activated

 Please wait while we load your content...
Please wait while we load your content...