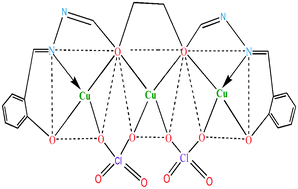
Three new homotrimetallic copper(II) complexes ([Cu3(Ln)(ClO4)2(H2O)m] with H4Ln = H4L1 − H4L3, m = 0, 3) have been synthesized from substituted succinoyldihydrazones (H4Ln) in methanol. The composition of the complexes has been established on the basis of data obtained from analytical and mass spectral studies and molecular weight determination in DMSO. The structure of the complexes has been discussed in the light of molar conductance, magnetic moment data and electronic, EPR, IR and FT-IR spectral studies. The molar conductance values for the complexes in the region of 1.2–1.7 Ω−1 cm2 mol−1 in DMSO indicate that the complexes are nonelectrolytes. The magnetic moment values for the complexes suggest considerable metal–metal interaction in the structural unit of the complexes. Copper centres have square planar and square pyramidal stereochemistry. The EPR parameters of complexes 1 and 3 indicate that the copper centre has a doublet ground state, while for complex 2, the ground state is a mixture of both doublet and quartet states. Electron transfer reactions of the complexes have been investigated by cyclic voltammetry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...