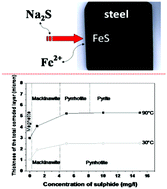
The influence of sulphide ion concentration on the behavior of carbon steel in a synthetic solution at 90 °C has been investigated using the methods of weight loss, scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS), confocal micro-Raman spectroscopy and X-ray diffraction (XRD). Corrosion batch experiments were conducted at 90 °C for 1 month with steel coupons immersed in Na2S solutions. Weight loss measurements revealed that the corrosion layer resistance is strongly dependent on both the sulphide concentration and the physicochemical properties of the corrosion products. In the absence of sulphide ions, a magnetite (Fe3O4) corrosion product layer was formed on the steel coupon, while in presence of sulphide ions (1 mg l−1), we observed the formation of a less protective mackinawite corrosion layer. At higher sulphide concentrations (5–15 mg l−1), highly protective pyrrhotite and pyrite are formed, inhibiting the steel corrosion process. Thus, it has been suggested that the formation of pyrite and/or pyrrhotite could be a promising strategy to protect against carbon steel corrosion in sulphidogenic media.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...