Fast and catalyst-free hetero-Diels–Alder chemistry for on demand cyclable bonding/debonding materials†
Abstract
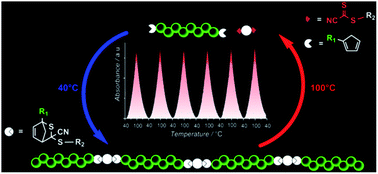
A new
* Corresponding authors
a
Preparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstr. 18, 76131 Karlsruhe, Germany
E-mail:
christopher.barner-kowollik@kit.edu
Fax: +49 721 608 45740
b Institut für Biologische Grenzflächen, Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
c Leibniz-Institut für Polymerforschung Dresden, Hohe Strasse 6, D-01069 Dresden, Germany
d Technische Universität Dresden, 01062 Dresden, Germany
e
ARC Centre of Excellence for Free-Radical Chemistry and Biotechnology, Research School of Chemistry, Australian National University, Canberra, ACT 0200, Australia
E-mail:
mcoote@rsc.anu.edu.au
f Evonik Industries AG, Rodenbacher Chaussee 4, 63457 Hanau-Wolfgang, Germany
g Evonik Industries AG, Paul-Baumann-Strasse 1, 45764 Marl, Germany
A new

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
K. K. Oehlenschlaeger, N. K. Guimard, J. Brandt, J. O. Mueller, C. Y. Lin, S. Hilf, A. Lederer, M. L. Coote, F. G. Schmidt and C. Barner-Kowollik, Polym. Chem., 2013, 4, 4348 DOI: 10.1039/C3PY00476G
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
