pH degradable dendron-functionalized poly(2-ethyl-2-oxazoline) prepared by a cascade “double-click” reaction
Abstract
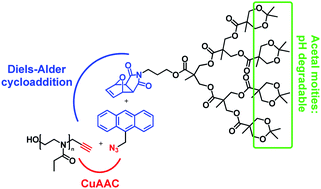
In this study, we report on the synthesis of a dendron-functionalized poly(2-ethyl-2-oxazoline) in a one-pot cascade reaction approach. By means of a bifunctional small organic compound linker, namely (9-azidomethyl)anthracene,

 Please wait while we load your content...
Please wait while we load your content...