Synthesis and structure of large difluoromethylene containing alicycles by ring closing metathesis (RCM)†
Abstract
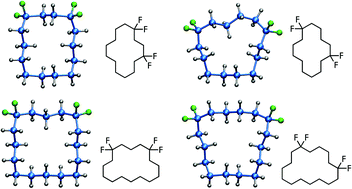
Cyclotetra- and cyclohexa-decane ring systems were prepared with CF2 groups spaced 1,4- and 1,6- for tetradecanes together with 1,5- and 1,6- for hexadecanes. These alicyclic systems were assembled by ring closing metathesis reactions of long terminal diolefins. Ring cyclisation by RCM was promoted by the introduction of the dithiane motif, using a sulfur compatible metathesis catalyst. This gave rise to macrocyclic E-cycloalkanes, which were hydrogenated also using a sulfur compatible catalyst. Finally the dithianes emerged as appropriate precursor motifs for the introduction of difluoromethylene groups. X-Ray structures revealed that the resultant rings have the CF2 groups located only at corner positions and that these groups dictated the overall macrocyclic ring conformations.

 Please wait while we load your content...
Please wait while we load your content...