The effect of the hydrophobic environment on the retro-aldol reaction: comparison to a computationally-designed enzyme†
Abstract
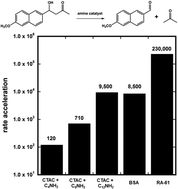
Recent work on a computationally-designed retroaldolase RA-61 suggested that most of the rate-acceleration brought about by this enzyme was due to non-specific interactions with the aromatic substrate. To provide a benchmark for the role of non-specific interactions in this system, we measured the second-order rate constant for the amine-catalysed retro-aldol reaction of methodol in the presence of non-specific hydrophobic pockets such as micelles. We found that a simple micellar system, that consists of a positively-charged surfactant and a long-chain amine, can accelerate the retro-aldol reaction of methodol by 9500-fold. This effect rivals the 105-fold rate acceleration of RA-61. Similar results were obtained with BSA used as the catalyst, implying that the retro-aldol reaction of methodol can be greatly accelerated by non-specific hydrophobic pockets that contain an amino group.

 Please wait while we load your content...
Please wait while we load your content...