An organocatalytic highly efficient approach to the direct synthesis of substituted carbazoles in water†
Abstract
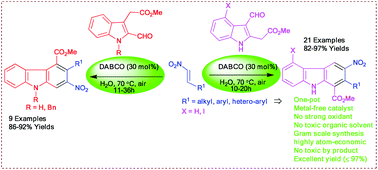
A simple, mild, green, catalytic and general procedure for the direct synthesis of highly functionalized 1-methoxycarbonyl-2-aryl/alkyl-3-nitro-9H-carbazoles has been achieved in water medium via a one-pot domino Michael–Henry/aromatization reaction of methyl 2-(3-formyl-1H-indol-2-yl)acetates with aryl/alky-substituted β-nitroolefins under air using DABCO (30 mol%) as an organocatalyst. In addition, the bench scale synthesis can be performed without using toxic organic solvents and a biologically important new fused carbazole has been prepared.

 Please wait while we load your content...
Please wait while we load your content...