Ternary alloyed AgClxBr1−x nanocrystals: facile modulation of electronic structures toward advanced photocatalytic performance†
Abstract
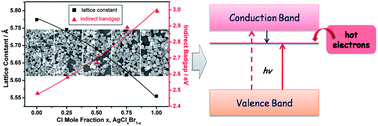
Manipulating the electronic structure of semiconductor photocatalysts represents an ideal approach for the exploration and development of photocatalysis. However, it still remains a challenge in terms of silver halide photocatalysts. Herein, we report ternary alloyed AgClxBr1−x nanocrystals (NCs) synthesized by controlling the crystal growth process within a facile microemulsion system. The alloyed NCs crystallize in a homogeneous rock-salt crystal structure and possess tunable bandgaps from 2.5 to 3.0 eV obtained by varying the halogen mole ratios (Cl/Br). Their photocatalytic activities for dye degradation and CO2 reduction are found to depend strongly on the chemical compositions, and among them, the AgCl0.75Br0.25 sample exhibits the highest activity (about 2–4 times higher than AgCl and AgBr). Further theoretical calculations demonstrate that a decrease of the ratios of Cl/Br lowers the levels of the conduction band minimum and thereby narrows the bandgaps. Combining the theoretical and experimental results, the highest activity can be rationally ascribed to the optimum conduction band levels, which balances the overall effect of bandgap, electronic coupling and redox potential. This methodological exploration of engineering the bandgap of silver halide materials is a step forward toward the development of advanced photocatalysts and will shed light on devising various semiconductor photocatalytic systems.

 Please wait while we load your content...
Please wait while we load your content...