Ethyl 2-hydroxy-2-methylpropanoate derivatives of magnesium and zinc. The effect of chelation on the homo- and copolymerization of lactide and ε-caprolactone†
Abstract
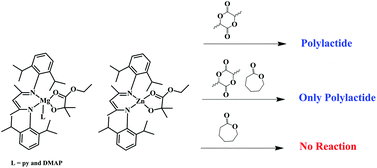
The preparation, single crystal and molecular structures of the compounds (BDI)Mg(OCMe2COOEt)(L) are described where BDI = 2-[(2,6-diisopropylphenyl)amino]-4-[(2,6-diisopropylphenyl)imino]pent-2-ene and L = pyridine (py) and 4-dimethylaminopyridine (DMAP). In the solid-state the molecules have five coordinate Mg2+ ions that may be considered as derived from a trigonal bipyramid where the chelating BDI and OCMe2COOEt ligands span equatorial-axial sites. IR spectroscopic data obtained in CH2Cl2 indicate that the chelating nature of the OCMe2COOEt ligand is maintained in solution and variable temperature NMR studies reveal that the ligands py or DMAP undergo a rapid reversible dissociation in toluene-d8 and especially in THF-d8. This leads to a facile exchange involving coordinated and free L upon the addition of L and evidence is presented to support the view that this occurs exclusively by a dissociative interchange mechanism. Zinc forms a related complex (BDI)Zn(OCMe2COOEt) lacking additional coordination of L but maintaining the chelating nature of the OCMe2COOEt ligand and undergoing reversible coordination of L in the presence of added ligands L. The magnesium and zinc complexes will initiate and sustain the living polymerization of lactide (L- or rac-LA) but not the ring-opening polymerization of ε-caprolactone.

 Please wait while we load your content...
Please wait while we load your content...