pH changes the aggregation propensity of amyloid-β without altering the monomer conformation†
Abstract
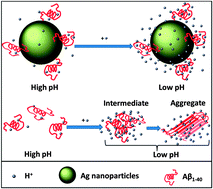
Decoupling conformational changes from aggregation will help us understand amyloids better. Here we attach Alzheimer's amyloid-β1–40 monomers to silver nanoparticles, preventing their aggregation, and study their conformation under aggregation-favoring conditions using SERS. Surprisingly, the α-helical character of the peptide remains unchanged between pH 10.5 and 5.5, while the solubility changes >100×. Amyloid aggregation can therefore start without significant conformational changes.

 Please wait while we load your content...
Please wait while we load your content...