pH sensitive tubules of a bile acid derivative: a tubule opening by release of wall leaves†
Abstract
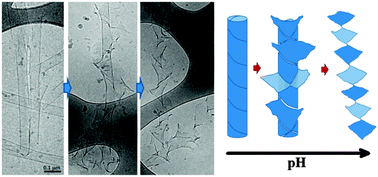
Tubules formed by
* Corresponding authors
a
Dipartimento di Chimica, Università di Roma “La Sapienza”, P. le A. Moro 5, 00185 Roma, Italy
E-mail:
luciano.galantini@uniroma1.it
b Universidad de Santiago de Compostela, Facultad de Ciencias, Avda. Alfonso X El Sabio s/n, 27002 Lugo, Spain
c Escuela de Química Universidad de Costa Rica, Centro de Electroquímica y Energía Química (CELEQ), San Josè, Costa Rica
d Department of Chemical Engineering and Ilse Katz Institute for Nanoscale Science and Technology, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel
e Division of Physical Chemistry, Department of Chemistry, Center of Chemistry and Chemical Engineering, Lund University, P.O. Box, SE-221 00 Lund, Sweden
Tubules formed by

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. C. di Gregorio, N. V. Pavel, A. Jover, F. Meijide, J. Vázquez Tato, V. H. Soto Tellini, A. Alfaro Vargas, O. Regev, Y. Kasavi, K. Schillén and L. Galantini, Phys. Chem. Chem. Phys., 2013, 15, 7560 DOI: 10.1039/C3CP00121K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
