Cu(i)-catalysed N–H insertion in water: a new tool for chemical biology†
Abstract
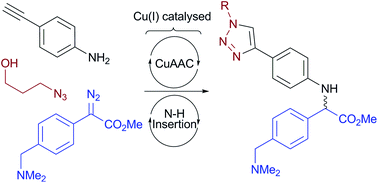
We demonstrate for the first time that Cu(I)-catalysis can deliver N–H insertion (NHI) with α-diazocarbonyl compounds in aqueous media. Despite being carried out in water only trace amounts of O–H insertion are seen, indicating the catalyst's overwhelming preference for NHI. Our optimized conditions for NHI converged with those used for the Cu-catalyzed azide–alkyne cycloaddition (CuAAC) for bioconjugation, spurring us to develop an auto-tandem catalytic process where both catalytic cycles operate simultaneously in one-pot. We explore the scope of the method with both small molecule and complex DNA and RNA substrates.

 Please wait while we load your content...
Please wait while we load your content...