Synthesis and biological evaluation of non-isomerizable analogues of Ala-tRNAAla
Abstract
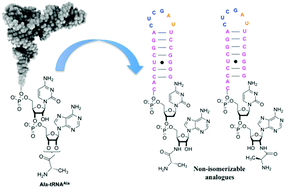
Aminoacyl-tRNAs serve as amino acid donors in many reactions in addition to protein synthesis by the ribosome, including synthesis of the peptidoglycan network in the cell wall of bacterial pathogens. Synthesis of analogs of aminoacylated tRNAs is critical to further improve the mechanism of these reactions. Here we have described the synthesis of two non-isomerizable analogues of Ala-tRNAAla containing an amide bond instead of the isomerizable ester that connects the amino acid with the terminal adenosine in the natural substrate. The non-isomerizable 2′ and 3′ regioisomers were not used as substrates by FemXWv, an alanyl-transferase essential for peptidoglycan synthesis, but inhibited this enzyme with IC50 of 5.8 and 5.5 μM, respectively.

 Please wait while we load your content...
Please wait while we load your content...