C–C bond fragmentation by Grob/Eschenmoser reactions, applications in dendrimer synthesis†
Abstract
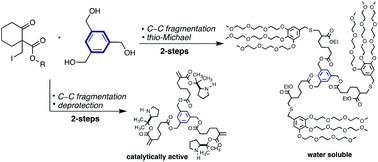
C–C bond fragmentation of structurally diverse carbocycles has been applied to the divergent synthesis of dendrimers. The fragmentation has been paired to deprotection or thio-Michael reaction, allowing the preparation of a fourth generation dendrimer of narrow molecular weight distribution. Methodologies to increase

 Please wait while we load your content...
Please wait while we load your content...