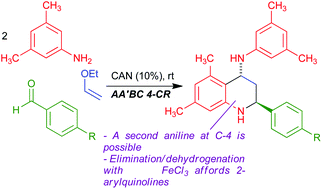
Diastereoselective, multicomponent access to trans-2-aryl-4-arylamino-1,2,3,4-tetrahydroquinolines via an AA′BC sequential four-component reaction and their application to 2-arylquinoline synthesis†
Abstract
The CAN-catalyzed reaction between 3,5-disubstituted

 Please wait while we load your content...
Please wait while we load your content...