Preparation and photophysical properties of a tetraethylene glycol-linked phthalocyanine–porphyrin dyad and triad
Abstract
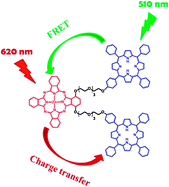
Treatment of 1,4-bis(tetraethylene glycol)-substituted zinc(II) phthalocyanine with p-toluenesulfonyl chloride and triethylamine followed by 5-(4-hydroxyphenyl)-10,15,20-triphenylporphyrin and K2CO3 resulted in the formation of both a covalently linked phthalocyanine–porphyrin dyad and triad. The photophysical properties of these hetero-arrays were studied in detail using steady-state and time-resolved spectroscopic methods. Upon excitation at the porphyrin moiety, both compounds exhibited predominantly highly efficient excitation energy transfer from the excited porphyrin to the phthalocyanine core. The rate of energy transfer (2.4 × 1010 s−1) was more than 20-fold faster than the rate of electron transfer (1.1 × 109 s−1). When the phthalocyanine core of the dyad and triad was excited either directly or via excitation energy transfer, its singlet excited state underwent charge transfer leading to reduction in the fluorescence quantum yield and fluorescence lifetime. The rate of charge transfer for the triad (2.8 × 108 s−1) was about two-fold higher than that for the dyad (1.5 × 108 s−1) due to the presence of an additional porphyrin moiety in the triad.
- This article is part of the themed collection: 崛起的中国—Hello from China!

 Please wait while we load your content...
Please wait while we load your content...