Catalytic etherification of glycerol with short chain alkyl alcohols in the presence of Lewis acids†
Abstract
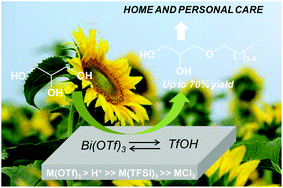
Here we report the homogeneously-catalyzed etherification of
* Corresponding authors
a
Institut de Chimie des Milieux et Matériaux de Poitiers, CNRS/Université de Poitiers/ENSIP, 1 rue Marcel Doré, 86022 Poitiers, France
E-mail:
francois.jerome@univ-poitiers.fr
Fax: +33 5 49 45 33 49
Tel: +33 5 49 45 40 52
b
Eco-Efficient Products and Processes Laboratory, Unité Mixte de Recherche UMI 3464 CNRS/RHODIA, 3966 Jin Du Road, Shanghai 201108, China
E-mail:
floryan.decampo@ap.rhodia.com
Fax: +86 21 54425911
Tel: +86 21 24089391
Here we report the homogeneously-catalyzed etherification of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
F. Liu, K. De Oliveira Vigier, M. Pera-Titus, Y. Pouilloux, J. Clacens, F. Decampo and F. Jérôme, Green Chem., 2013, 15, 901 DOI: 10.1039/C3GC36944G
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
