Salt melt synthesis of ceramics, semiconductors and carbon nanostructures
Abstract
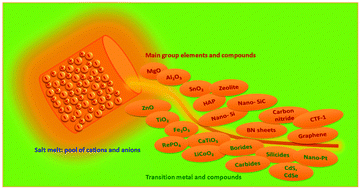
Materials synthesis in the liquid phase, or wet-chemical synthesis, utilizes a solution medium in which the target materials are generated from a series of chemical and physical transformations. Although this route is central in organic chemistry, for materials synthesis the low operational temperature range of the solvent (usually below 200 °C, in extreme 350 °C) is a serious restriction. Here, salt melt synthesis (SMS) which employs a molten inorganic salt as the medium emerges as an important complementary route to conventional liquid phase synthesis. Depending on the nature of the salt, the operational temperature ranges from near 100 °C to over 1000 °C, thus allowing the access to a broad range of inorganic crystalline materials and carbons. The recent progress in SMS of inorganic materials, including oxide ceramic powders, semiconductors and carbon nanostructures, is reviewed here. We will introduce in general the range of accessible materials by SMS from oxides to non-oxides, and discuss in detail based on selected examples the mechanisms of structural evolution and the influence of synthetic conditions for certain materials. In the later sections we also present the recent developments in SMS for the synthesis of organic solids: covalent frameworks and polymeric semiconductors. Throughout this review, special emphasis is placed on materials with nanostructures generated by SMS, and the possible modulation of materials structures at the nanoscale in the salt melt. The review is finalized with the summary of the current achievements and problems, and suggestions for potential future directions in SMS.

 Please wait while we load your content...
Please wait while we load your content...