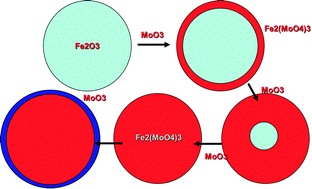
Iron molybdate catalysts are used for the selective oxidation of methanol to formaldehyde. In this paper we have attempted to understand what determines high selectivity in this reaction system by doping haematite with surface layers of Mo by incipient wetness impregnation. This works well and the Mo appears to form finely dispersed layers. Even very low loadings of Mo have a marked effect on improving the selectivity to formaldehyde. Haematite itself is a very poor catalyst with high selectivity to combustion products, whereas, when only 0.25 monolayers of Mo are deposited on the surface, formaldehyde and CO selectivities are greatly enhanced and CO2 production is greatly diminished. However, even with as much as seven monolayers of Mo dosed on to the surface, these materials achieve much less selectivity to formaldehyde at high conversion than do the industrial catalysts. The reason for this is that the Mo forms a ‘skin’ of ferric molybdate on a core of iron oxide, but does not produce a pure Mo oxide monolayer on the surface, a situation which is essential for very high yields of formaldehyde.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...