In situ ATR-IR observation of nucleation and crystal growth of KH2PO4 in aqueous solution
Abstract
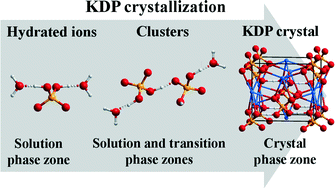
In situ Attenuated Total Reflectance-Infrared (ATR-IR) spectroscopy was used to record and characterize the nucleation and crystal growth of KH2PO4 (KDP) from aqueous solution with pH = 1.01–6.96. In the crystallization of KDP, K+ and H2PO4− ions transform from hydrated ions to clusters and crystalline state. With increasing measurement time, the shift of v3(PO4) at 938 and 1145 cm−1 towards lower wavenumbers illustrated the breaking of hydrogen bonding between H2PO4− and H2O. IR bands of vO–H at 2370 cm−1 and βO–H at 1274 cm−1 indicated the formation of H2PO4− clusters possessing KDP lattice structural characteristics via hydrogen bonding. The disappearance of v3(PO4) at 1145 cm−1 indicated the chemical bonding between K+ and H2PO4− in the nucleation stage of KDP. In KDP supersaturated solution with pH = 2.02–6.96, H2PO4− groups with larger hydrated ionic radius initially form a framework structure, and then K+ ions with a relatively smaller hydrated ionic radius insert into the H2PO4− framework to form KDP. Moreover, the time needed for the appearance of KDP solids in IR measurement can be modified by varying the pH value of KDP supersaturated solution. Such an in situ visualization strategy can characterize the structural variations by spectroscopy, which deepens the insight into the nucleation and crystal growth from aqueous solution during the whole crystallization process.

 Please wait while we load your content...
Please wait while we load your content...