Tuning the Zn(ii) coordination assembly by adjusting the spacers of 2-pyridylthiomethyl functionalized 1,2,3-triazoles†
Abstract
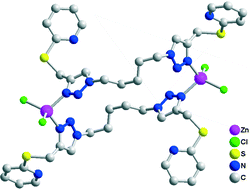
Three novel pyridyl and thioether functionalized bis-chelating 1,2,3-triazoles bis(4-(2-pyridylthiomethyl)-1H-1,2,3-triazolyl)alkane (alkane = ethane (L1), butane (L2) and pentane (L3)), as well as bridging 1-(3-picolyl)-4-(2-pyridylthiomethyl)-1H-1,2,3-triazole (L4) and 1-(4-picolyl)-4-(2-pyridylthiomethyl)-1H-1,2,3-triazole (L5) have been synthesized from CuAAC reactions. Their coordination with Zn(II) gives dinuclear [Zn2Cl4(L1)] (1), [Zn2Cl4(L2)] (2), [Zn2Cl4(L3)2] (3), [Zn2Cl4(L5)2] (5) and polymeric [ZnCl2(L4)]n (4) photoluminescent complexes. In 1 and 2, a single ligand connects and chelates to two metal centers through its triazolyl–pyridyl nitrogen donors. In 3 and 5, two ligands co-bridge to give metallocyclic cavities. Polymer 4 shows a 1-D spiral propagation assembled by a triazole with distal 3-picolyl nitrogen. It is further stabilised by orthogonal inter-chain π⋯π stacking interactions to give a 2-D network. All 3–5 materials carry uncoordinated thiopyridyl pendants. Formation of these assemblies suggest that hybrid spacers with three functional units, viz. triazole, picolyl and thiopyridyl, demonstrate coordinative and skeletal flexibilities to determine the assembly outcome. Both metallocyclic and polymeric assemblies can be supported.

 Please wait while we load your content...
Please wait while we load your content...