Solid-state photodimerization reactions of racemic and homochiral phenylalanine sulfonamidecinnamic acids†
Abstract
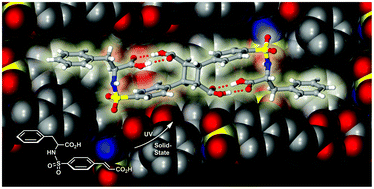
Racemic and enantiopure phenylalanine sulfonamides preferentially crystallize to give supramolecular dimers with favorable olefin⋯olefin spacing (3.77–4.01 Å) for programmed solid-state [2 + 2] photodimerizations.

 Please wait while we load your content...
Please wait while we load your content...