Deracemization and the first CD spectrum of a 310-helical peptide made of achiral α-amino-isobutyric acid residues in a chiral membrane mimetic environment†‡
Abstract
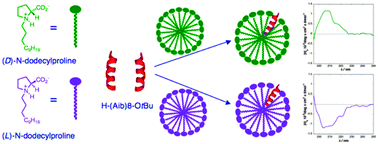
Interaction of the racemic helical homo-octapeptide made by the achiral Cα-methyl alanine (Aib) amino acid with a chiral enantiopure micellar aggregate made of N-dodecylproline led to the deracemization of the helical Aib sequence thus allowing us to obtain for the first time the CD signature in water of a 310 helix devoid of the contribution of any chiral amino acid.

 Please wait while we load your content...
Please wait while we load your content...