Reconstitution of the pyridoxal 5′-phosphate (PLP) dependent enzyme serine palmitoyltransferase (SPT) with pyridoxal reveals a crucial role for the phosphate during catalysis†‡
Abstract
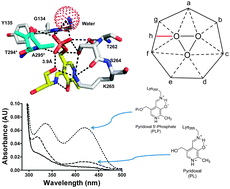
The pyridoxal 5′-phosphate (PLP)-dependent enzyme serine palmitoyltransferase (SPT) is required for de novo sphingolipid biosynthesis. A previous study revealed a novel and unexpected interaction between the hydroxyl group of the L-serine substrate and the 5′-phosphate group of PLP. By using pyridoxal (PL), the dephosphorylated analogue of vitamin B6, we show here that this interaction is important for substrate specificity and optimal catalytic efficiency.

 Please wait while we load your content...
Please wait while we load your content...