Reduction-sensitive core-cross-linked mPEG–poly(ester-carbonate) micelles for glutathione-triggered intracellular drug release†
Abstract
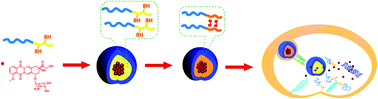
A functionalized six-membered cyclic carbonate monomer containing a protected thiol group, 2-(2,4-dinitrophenylthio)ethyl-5-methyl-2-oxo-1,3-dioxane-5-carboxylate (MTC), was prepared. Ring-opening polymerization (ROP) of MTC and L-lactide (LA) initiated by monohydroxyl poly(ethylene glycol) (mPEG) was successfully performed with controlled polymer molecular weight and molecular weight distribution. The structures of the obtained monomer and copolymers were confirmed by NMR and FTIR. After deprotection under mild conditions, the amphiphilic block copolymer was self-assembled into micelles in aqueous solution. By oxidation of the free thiol groups in the PMTC segments, the micelles with the cross-linked core were formed, and the enhanced stability against disruptive conditions was demonstrated by dynamic light scattering (DLS) measurement in the presence of DMF. Doxorubicin (DOX) was loaded into the micelles as a model drug. The in vitro DOX release behaviors indicated that cross-linking of the micelle cores resulted in a slow drug release and this process was greatly accelerated by adding glutathione (GSH) solution with comparable concentrations to intracellular levels in tumor cells. The cross-linked micelles were demonstrated to be efficiently internalized into the cells. Faster intracellular DOX release was observed by confocal laser scanning microscopy (CLSM) in the GSH pretreated MCF-7 cells than in the unpretreated MCF-7 cells. In vitro MTT assay revealed that the cross-linked micelles were biocompatible with L929 cells and MCF-7 cells. As expected, DOX-loaded cross-linked micelles showed higher cellular proliferation inhibition towards glutathione pretreated MCF-7 cells than that towards the unpretreated ones at various GSH concentrations. These results indicated that the bioreducible core-cross-linked micelles can be used as drug carriers for intelligent drug delivery.

 Please wait while we load your content...
Please wait while we load your content...