Solvent-free double aza-Michael under ultrasound irradiation: diastereoselective sequential one-pot synthesis of pyrrolidineLobeliaalkaloids analogues†
Abstract
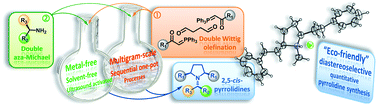
Novel 2,5-meso-pyrrolidines have been straightforwardly synthesized from readily available symmetrical double Michael acceptors. The key step rested on an aza-Michael addition of primary

 Please wait while we load your content...
Please wait while we load your content...