Using light and a molecular switch to ‘lock’ and ‘unlock’ the Diels–Alder reaction†
Abstract
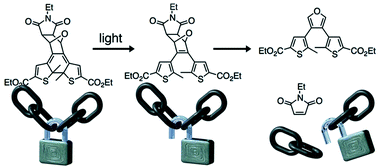
Light is used to ‘gate’ the Diels–Alder reaction using a photoresponsive dithienylfuran backbone and turn the reversibility of the Diels–Alder reaction ‘off’ and ‘on’ at 100 °C. These features make the reported system an excellent candidate for developing the next generation of self-healing
 Please wait while we load your content...
Please wait while we load your content...