Mechanism and optimisation of the homoboroproline bifunctional catalytic asymmetric aldol reaction: Lewis acid tuning through in situ esterification†‡
Abstract
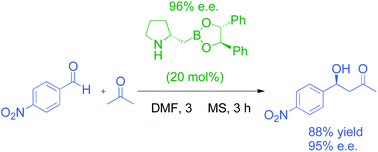
The use of homoboroproline as a bifunctional catalyst in the asymmetric aldol reaction has been investigated mechanistically, particularly with respect to tuning the Lewis acidity of boron by in situ esterification with mildly sigma-electron withdrawing diols such as hydrobenzoin and tartrate esters. The stability of simple cyclohexyl and cyclopentyl boronate diol esters shows that the 5-ring boronate esters are more stable, which sheds light on the mode of action of esterified homoboroproline catalyst in the enamine-mediated aldol reaction, which is also studied by NMR. The result is reaction optimisation to provide an efficient aldol reaction and a proposed mechanistic proposal.
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...