A solid-state study of the inclusion of endosulfan in native and derivatised cyclodextrins using X-ray diffraction and thermoanalytical methods†
Abstract
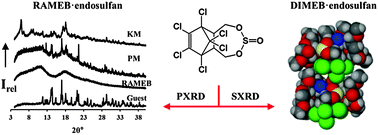
Endosulfan is a highly toxic insecticide used in several countries and known to be relatively persistent in the environment. This recently banned agrochemical is capable of forming solid-state inclusion complexes with both native and derivatised cyclodextrins (CDs). Differential scanning calorimetry and powder X-ray diffraction (PXRD) techniques were used to confirm complexation of β-CD, γ-CD and randomly methylated β-CD (RAMEB) with the technical 7α : 3β isomeric sample of endosulfan. The PXRD traces obtained for the inclusion complexes of the native CDs (β-CD and γ-CD) with endosulfan match those of other isostructural CD inclusion complexes. The single crystal X-ray structure of the complex formed between heptakis(2,6-di-O-methyl)-β-CD (DIMEB) and the β-endosulfan isomer is presented and shown to have a novel packing arrangement. The implications of the encapsulation of endosulfan in CDs are discussed with reference to the potential for sequestering the insecticide from contaminated areas.

 Please wait while we load your content...
Please wait while we load your content...