Efficient synthesis of unsymmetrical S-(bromodifluoromethyl)diarylsulfonium salts for electrophilic bromodifluoromethylating reagents†
Abstract
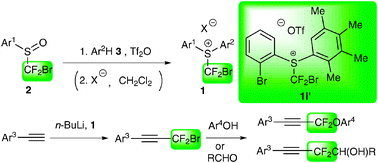
A series of unsymmetrical S-(bromodifluoromethyl)diarylsulfonium salts 1 were readily synthesized by treatment of corresponding (bromodifluoromethyl)arylsulfoxides 2 and substituted

 Please wait while we load your content...
Please wait while we load your content...